Riddled With Tungsten by Mistake in a Study
Women participating in a study of patients with breast cancer have been inadvertently left with hundreds of tiny particles of the heavy metal tungsten in their breast tissue and chest muscles. The particles came from a device used during surgery. The device has since been recalled.
Related
- Health Guide: Breast Cancer
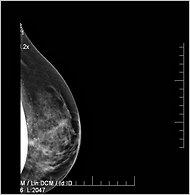
An X-ray showing tiny particles of tungsten in breast tissue.
It is not known if the metal is dangerous to health because relatively little research has been done on its long-term effects in the body. But it shows up on mammograms, and may make them difficult to read, an especially troubling effect for women who have already had breast cancer and worry about recurrences. (The particles resemble calcium deposits, which can indicate cancer.)
About 30 women have been affected, according to the manufacturer of the device that caused the problem, the Axxent FlexiShield Mini. The women are in a quandary. At least one, fearing that the tungsten could cause cancer or another illness, is trying to decide whether to get rid of the particles by having her breast and its underlying tissue removed in a radical and disfiguring operation.
Twenty-seven of the cases occurred at Hoag Memorial Hospital Presbyterian in Newport Beach, Calif. Eleven of those women have had mammograms, and all 11 showed tungsten. Hospital officials declined interviews, but issued a statement acknowledging that the problem had occurred.
Two other women were treated in a study at Karmanos-Crittenton Cancer Center in Rochester Hills, Mich. A hospital spokeswoman said that both patients had been informed of the recall and the potential problem but had not returned to the hospital.
The episode casts doubt on the safeguards for people who participate in medical research and on the Food and Drug Administration s ability to protect the public from flawed medical devices.
The Axxent FlexiShield Mini had been cleared by the agency in June 2009 in an abbreviated process used for devices that are considered equivalent to products already on the market. That process, known as 510(k), takes less time than the procedure used to approve a new device, and it generally does not require tests on humans. The FlexiShield Mini equipment was recalled last month. Neither its manufacturer nor the F.D.A. could explain what went wrong with the device.
Karen Riley, a spokeswoman for the agency, said it was just beginning its review of the device and the recall. So far, she said, F.D.A. toxicologists had found no evidence that the tungsten was toxic or that patients were harmed.
Ms. Riley said the 510(k) process was used to avoid reinventing the wheel for products that were essentially the same as others that had already passed muster with the agency.
The women who were exposed to the tungsten were taking part in a study of a radiation technique that some doctors predicted would be a big advance in the treatment of breast cancer. Unlike the usual five to seven weeks of daily radiation sessions, the newer method delivers the entire course of treatment in one dose while the woman is still in the operating room after undergoing a lumpectomy for breast cancer.
But in the study, a device that was temporarily placed in the women s incisions during the radiation treatment was apparently flawed, and riddled their breasts with tungsten. The Axxent Flexishield Mini, a $100 disk made of tungsten and silicone, was used to shield healthy tissue from the radiation.
The first patient to take part in the study at Hoag said the events had shattered her faith in the vigilance of the drug agency, the hospital and her surgeon, who she said enthusiastically talked her into participating, emphasizing how convenient it would be to finish radiation treatment before she even woke from surgery.
I do work, so it was appealing, said the woman, a 57-year-old psychologist with a busy practice who did not want her name used for privacy reasons.
The purpose of the study was not to test the new radiation treatment itself, but rather to determine whether imaging studies could correctly predict which women would be candidates for it. The device s manufacturer did not pay for the study.
It never occurred to the first patient that the equipment might be faulty, she said, because she knew that it had been approved by the F.D.A. She also trusted the doctor and hospital to ensure that the study was safe.
I had this illusion, like most people do, that the F.D.A. wouldn t allow this to happen, she said. I definitely feel like a lab rat now.
The manufacturer, Xoft, which was bought in December by iCad, intended the shield to be used with its portable radiation device, the Axxent Electronic Brachytherapy System.
The president of iCad, Ken Ferry, said his company bought Xoft because the idea of giving radiation treatment during surgery seemed so promising. A study published last summer showed good results from a different radiation machine using the same technique. Mr. Ferry said he thought the procedure might eventually be used to treat half of the 270,000 women a year in the United States who develop breast cancer.
We think the growth of the procedure will be dramatic over the next three years, Mr. Ferry said. That s what really drove us to acquire the company.
But iCad also acquired the tungsten problem, which became apparent only a week or two after the deal was closed.
If you need any more details of the above news and/or products, please visit Chinatungsten Online, or contact us directly.
Disclaimer: The article is only reflecting the opinions of the author. We have no responsibility to prove the originality and authenticity of the content, words and/or pictures. You readers should just take it as reference and check the details by yourselves. And the content is not a suggestion for investment decision. The investor takes his or her own risks if he or she operates accordingly. If you have any dissent about the contents above, please contact the relevant author, or the webmaster. We will try our best to assist the dealing of the related issues. Thanks for your visit and cooperation.





