Materials chemists in China have developed a compound that they believe should improve the quality of field emission displays (FEDs), bringing applications a step closer. FEDs have, for a number of years, been a promising technology for flat panel displays, but progress has been hampered by the display quality.
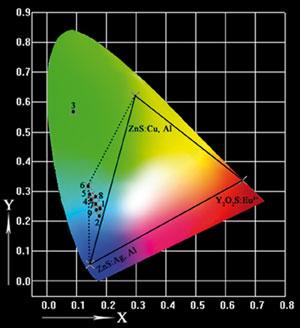 |
| The triangle and quadrangle show the typical and enlarged colour range for FED phosphors, respectively (Image by LIN Jun) |
FEDs have numerous advantages over other flat panel technologies, such as the plasma and liquid crystal displays commonly used in TVs and computer monitors. These advantages include lower energy consumption, wide viewing angle, good contrast ratio and wide working temperature range. However, the phosphors for FEDs need to work at lower voltages than those in cathode ray tubes, and developing low voltage phosphors with suitable colour characteristics has hindered uptake of the technology.
The phosphor developed by Lin's team is based on Mg2SnO4, previously known as a good base material for phosphors. By doping it with Mn2+ and Ti4+, they created a phosphor with a pure blue colour that works at low voltages. Lin says that the colour emitted is out of the range of the FED phosphors developed so far, giving it 'potential to increase the display quality of full-colour FEDs'. Importantly, he adds, the colour could be adjusted from green to blue simply by changing the concentrations of the two dopants.
Lin concludes that 'FEDs have been recognised as one of the most promising technologies in the flat panel display market,' and that improvements such as theirs will 'make the technology more viable in the future'.
Markku Leskel , an inorganic chemist at the University of Helsinki, Finland, says that 'blue colour is one of the limiting factors in fabrication of full-color field emission displays, and this is an interesting new material, which has potential.' However, he also notes that the stability would need to be improved for practical applications. 'The 8 per cent decrease in intensity observed over one hour would be a problem in real devices,' he says. (Chemistry World)
Related News
Photos
More>>trade
- NBPC Office Holds a Special Lecture on UN Convention Against Corruption and its
- National Experience-Sharing Conference on Deepening Government Affairs Publicity
- The Second Conference of States Parties to the United Nations Convention against
- Representatives of the Workshop of Corruption Prevention for Asian and African
- The Workshop of Corruption Prevention for Asian and African Countries Came Back
market
- He Yong Met With Representatives of the Workshop of Corruption Prevention for
- The Workshop of Corruption Prevention for Asian and African Countries Successful
- The First Phase of Special Discussion of the Workshop of Corruption Prevention
- Students of the Workshop of Civil Servants of Hong Kong Visited NBCP
- Fujian convenes working conference on corruption prevention
finance
- National Roving Seminar on PCT Held in Xi'an
- Shaanxi IP Administration Launches Patent Administrative Law-Enforcement
- Chinese-Foreign Symposium on "IPR System and Protection" Held in Ji'nan
- Guangzhou's First District-Level IPR Protection Association Established
- China International Industrial Design Forum Held in Wuxi





